

- #Viewing multiple unit cells in crystalmaker for mac#
- #Viewing multiple unit cells in crystalmaker movie#
- #Viewing multiple unit cells in crystalmaker free#
- #Viewing multiple unit cells in crystalmaker mac#
Featuring spectacular photo-realistic graphics and breathtaking 3D visualization, CrystalMaker excels at publication-quality graphics, without sacrificing ease-of-use.
#Viewing multiple unit cells in crystalmaker mac#
Whilst the majority of the screenshots are for the Mac version, both programs share the same feature set, albeit with different interfaces, tuned for their respective operating systems.ĬrystalMaker Interactive Crystal Structures VisualizationĬrystalMaker is a program for building, displaying and manipulating all kinds of crystal and molecular structures.
#Viewing multiple unit cells in crystalmaker for mac#
This overview covers both CrystalMaker for Mac (version 8.3) and CrystalMaker for Windows (version 2.3). Operating System Integration Mac System Integration Windows System Integrationĭiffraction Support CrystalDiffract Features SingleCrystal Features Outstanding Display Easy structure building Built-in model types Customizable model options Element colours and radius tables Bond and polyhedra generation Molecular crystal tools Customizable rendering options Depth profiling for massive/dense structuresįully-Interactive Extensive range of screen tools Instantly hide/show groups of atoms Molecule–Crystal conversion Place different structures into same model Easy surface/slab creation Supercell generation
#Viewing multiple unit cells in crystalmaker movie#
Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.CrystalMaker Product Overview November 2010ĬrystalMaker 8.3 for Mac CrystalMaker 2.3 for WindowsĬrystalMaker The CrystalMaker Advantage What’s New in CrystalMakerĪ Seamless Workflow Automatic Detection of File Formats Support for multi-structure text files Flexible interface for single and multi-structure working Animated Views with movie output Wide-ranging data export options Video recording Automatic generation of QTVR objects Outstanding, high-resolution graphics export is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Then you can share it with your target audience as well as ’s millions of monthly visitors. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. You might even have a presentation you’d like to share with others.
#Viewing multiple unit cells in crystalmaker free#
And, best of all, it is completely free and easy to use. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. is a leading presentation sharing website. half the cubic center sites areĬoordination no. Since stiochiometry of cation and anion 11,8Ĭs ions will fit into the cell.i.e. The centre of the cube is larger than theĮach unit cell has 8 anionsand 8 cubic centre ? Anions occupy the corners of a unit cell, (Cations fills in the diagonally opposite sites Since stiochiometry ofcation and anion 11,4Ĥ S2- packed in FCC,Zn2 will fit into the
Holes available in FCC unit cell closed packed ? cation would not be in contact with the ? the cation and anion remain in contact, but If the cation is larger than the ideal radius The radius ratio ( rcation / ranion) radiusįor a stable coordination, the bonded cation andĪnion must be in contact with each other. Octahedral or Tetrahedral hole? determined by (b) tetrahedral hole - coordinated by 4 anions (a) octahedral hole - coordinated by 6 anions
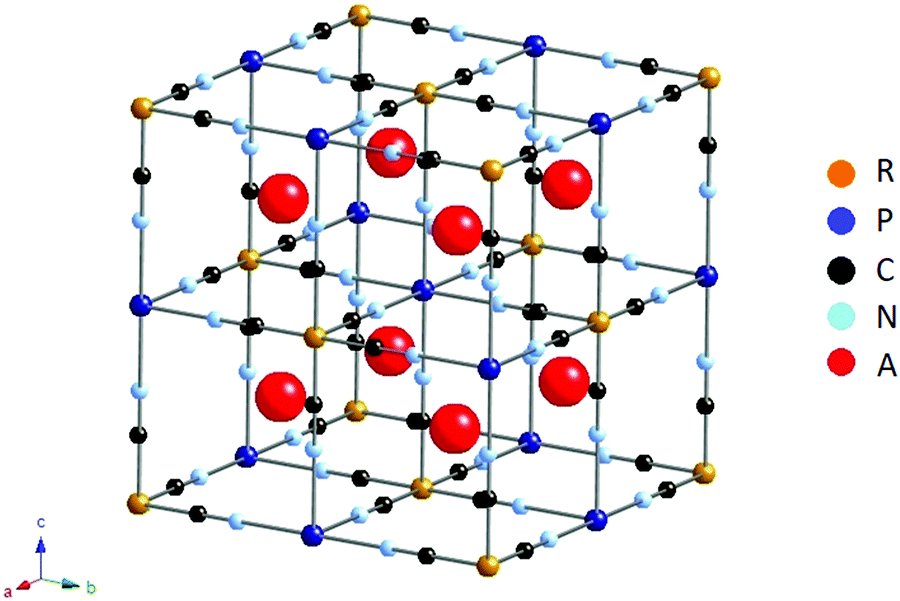
Stacking of two closed packed anion layers Governed by the radius ratio of cation and The number of nearest neighbor ions of oppositeĬharge is called the coordination number. With the cations occupying the vacant sites Packed arrangement of the larger anions (FCC or To visualize the structures in terms of a closed How do the anion and cationpack together? Many cations around each anion as is possible.īut it depends on the relative size of cation and Packing as many cations around each anion, and as The bonding forces should be maximized by Togetherin three dimensions, can generate the A unit cell is the smallest basic portion of the


 0 kommentar(er)
0 kommentar(er)
